4. A container contains 32 g of O2 at a temperature TThe pressure
4.9 (270) In stock
4.9 (270) In stock
4. A container contains 32 g of O2 at a temperature TThe pressure of the gas is P. An identical containercontaining 4 g of H2 at a temperature 2T has apressure of(1) 8P(3) P(2) 4P(4) P18r cnstant
4- A container contains 32 g of O2 at a temperature TThe pressure of the gas is P- An identical containercontaining 4 g of H2 at a temperature 2T has apressure of-1- 8P-3- P-2- 4P-4- P18r-cnstant

Solved 1. A container with a volume of 6.0 L holds 10 g of

A container of 5 i has a gas p=0.87 mathrm{m} of mathrm{Hg}_{32} tols is joint to an evaguent conlainere 3,-frac{331}{0.8} mathrm{m} capiaty. The rementing pressuren p=8 mathrm{g} mathrm{Hg} begin{aligned} r & text {

A container contains 32 g of O2 at a temperature T. The pressure of th

Solved (7) Consider the following statements: Consider the

A sealed container contains a mixture of oxygen and nitrogen gas.The ratio is .The ratio is
What is the amount of oxygen gas (O2) in a cylinder of 30l, if the pressure is 20atm at 30°C? - Quora

toppr-doubts-media.s3.aws.com/images/1818262

SOLVED: A vessel contains 1 mole of O2 gas (relative molar mass 32 ) at a temperature T . The pressure of the gas is P . An identical vessel containing 1
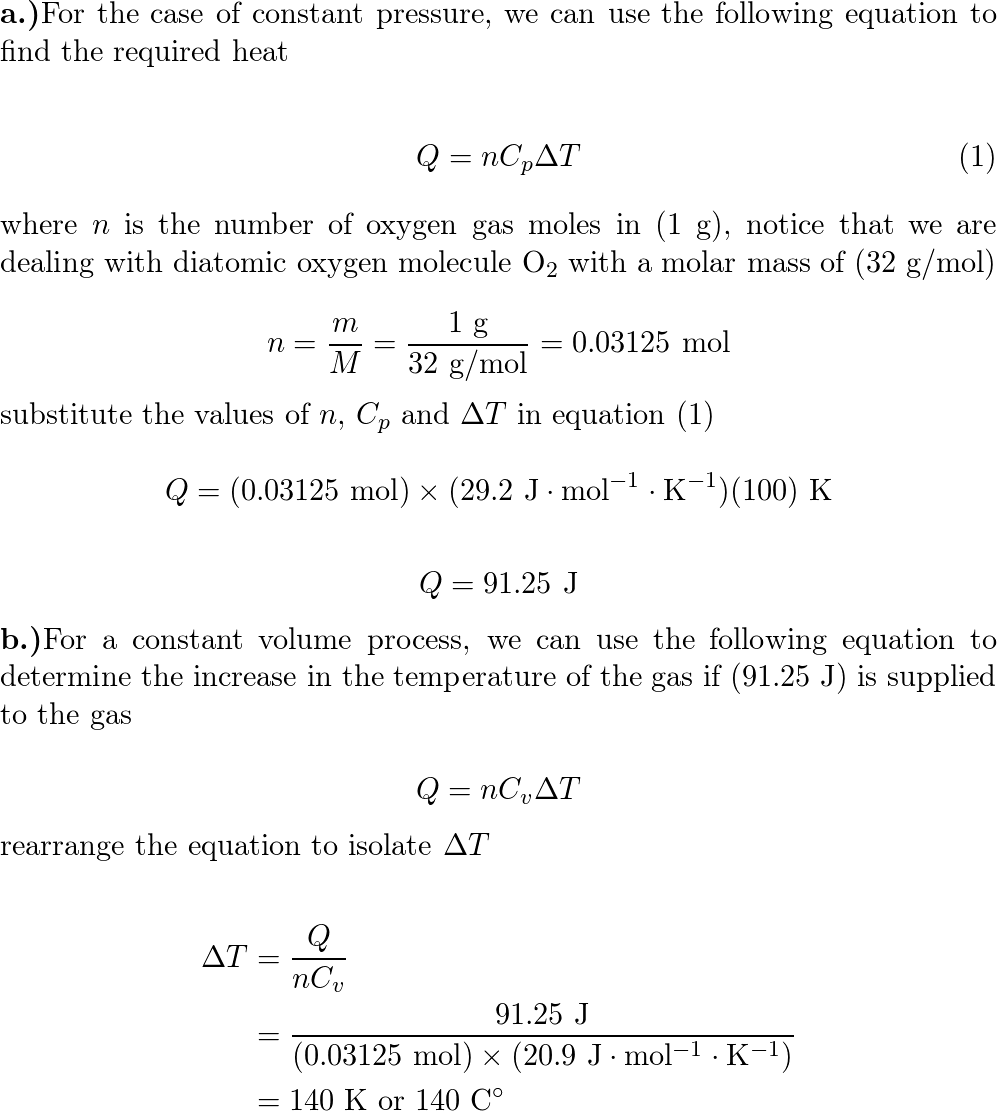
A container holds 1.0 g of oxygen at a pressure of 8.0 atm.

toppr-doubts-media.s3.aws.com/images/1948236
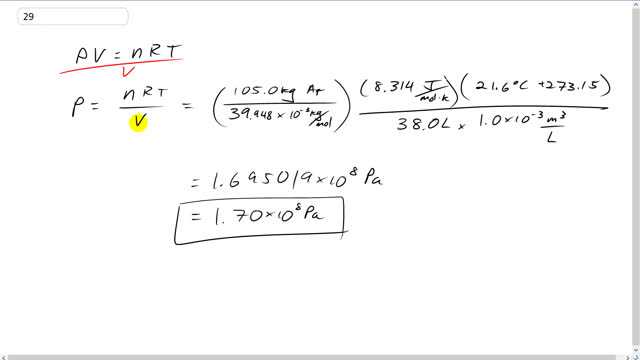
Giancoli 7th Edition, Chapter 13, Problem 29
A vessel contains equal number of hydrogen and oxygen molecules at one atmospheric pressure. The avg number of collisions per second at the walls of the vessel by hydrogen molecules is how