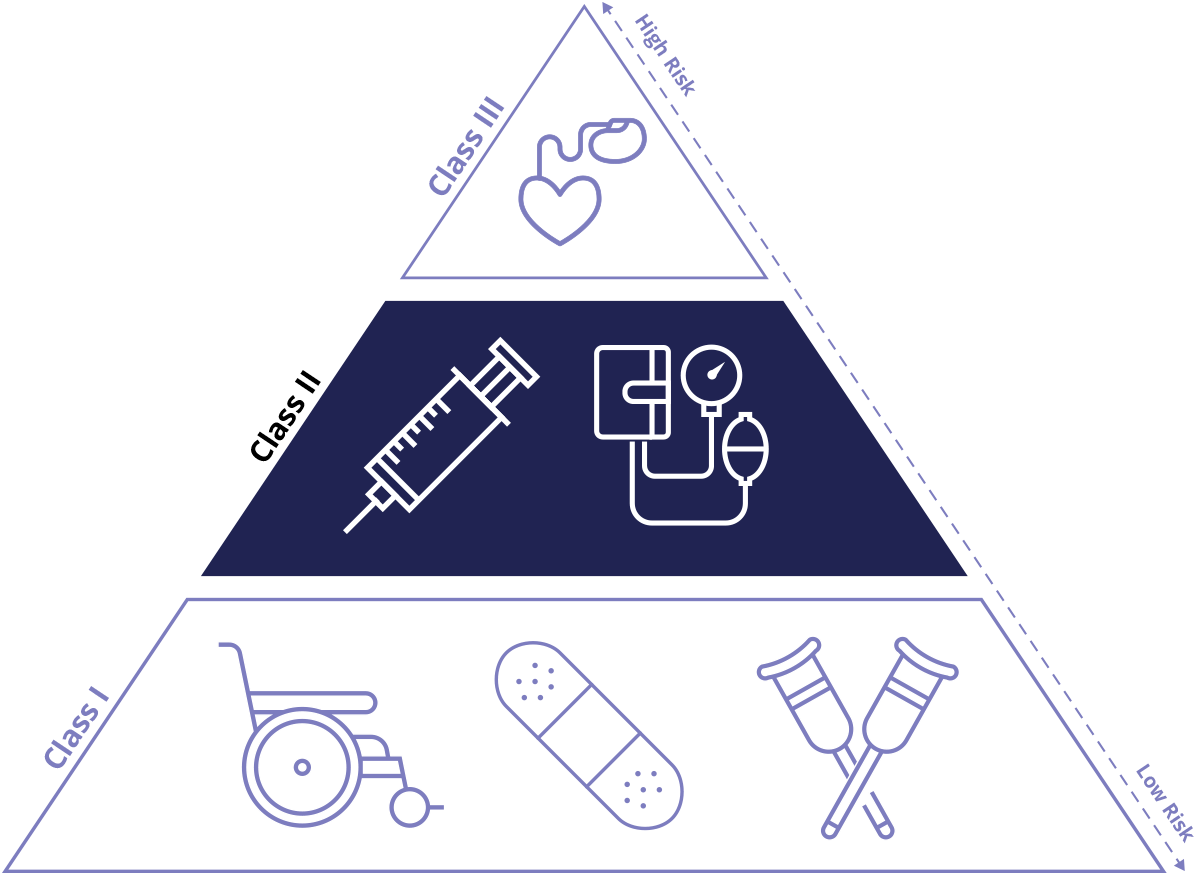
Class II Device Definition
4.9 (279) In stock

4.9 (279) In stock
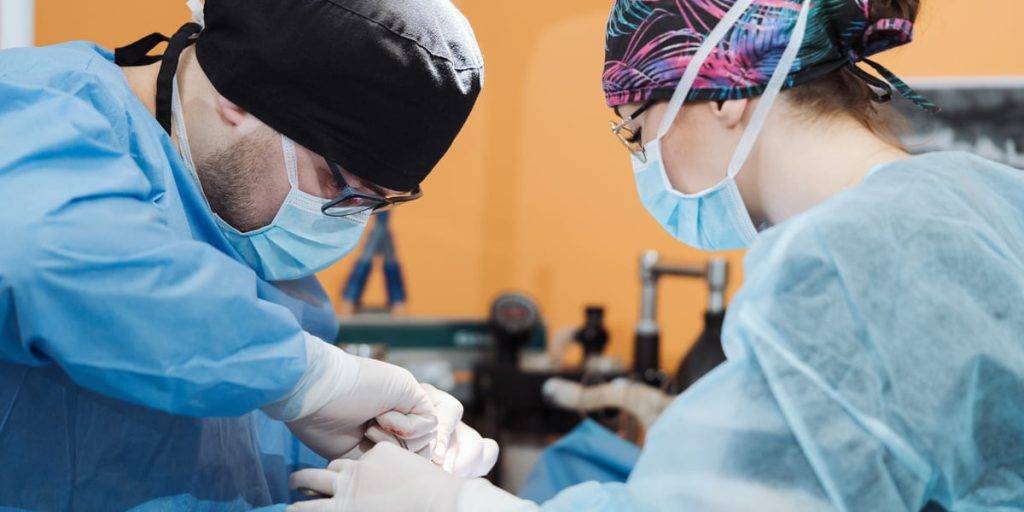
Class II medical devices have moderate to higher risks to patients or users. Over 40% of medical devices fall into this device category. The majority of medical devices are considered to be Class II devices. Some examples of Class II devices include catheters, syringes, contact lens, and pregnancy test kits.

Medical Device Manufacturing

Never accept the mark of the beast
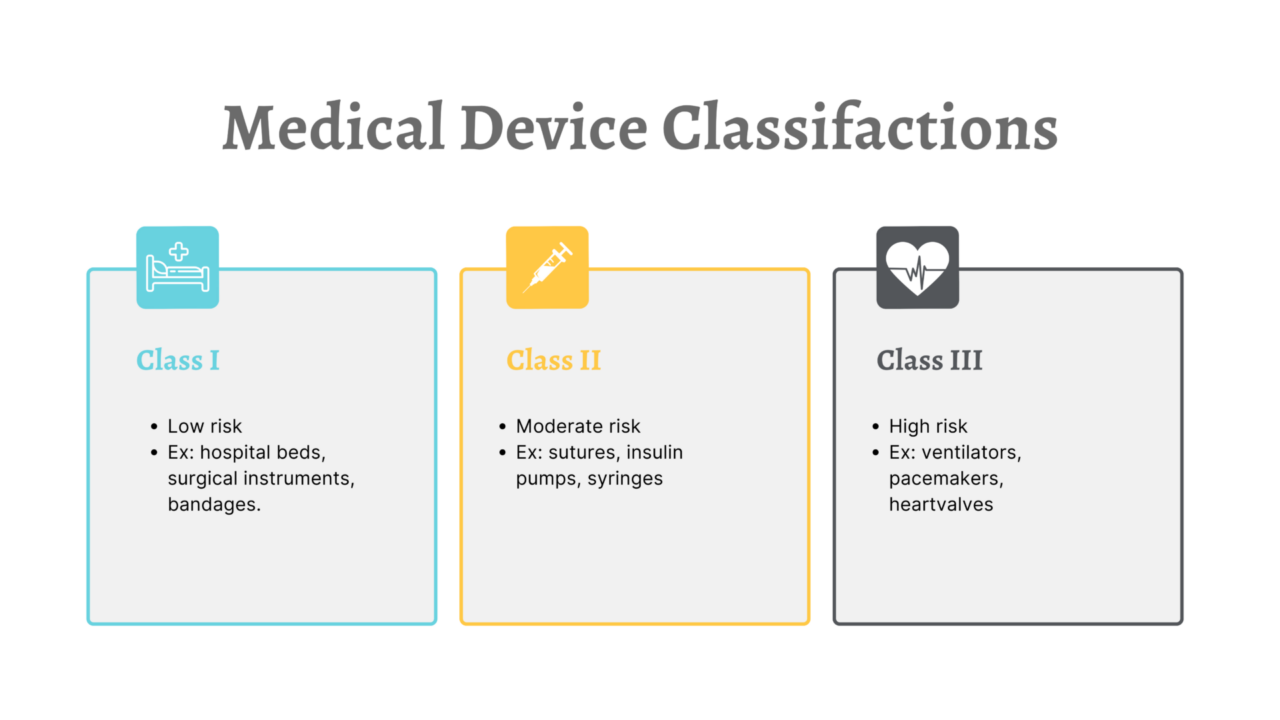
510(k) Exempt Medical Devices - Overview
Canada Medical Device Market Overview

What's the Difference between a Class I Medical Device and a Class II?
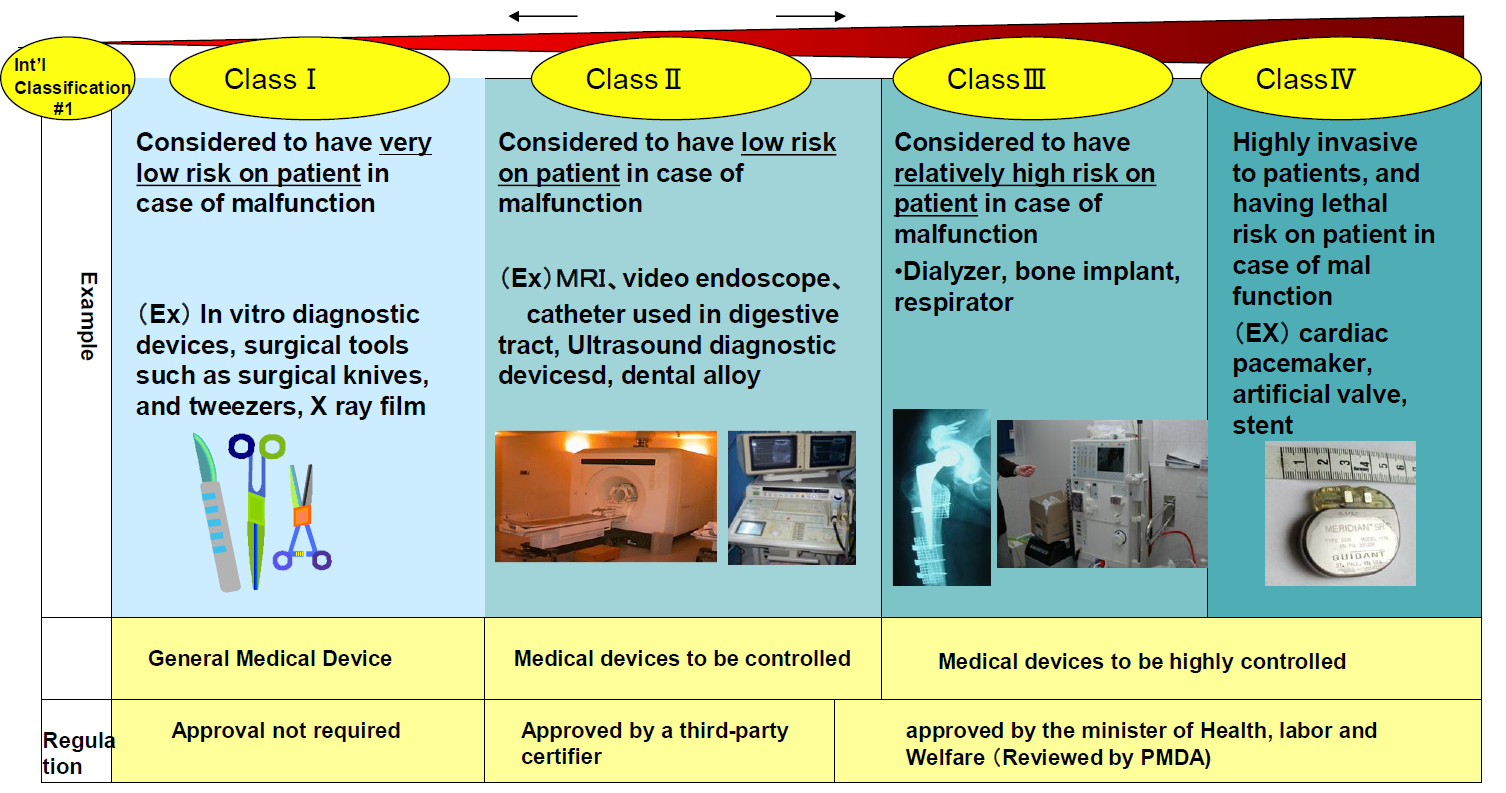
Interoperability standards for medical device integration in the OR and issues relating to international approval procedures (part 4) - ISCASBlog

Medical Devices Classification EU - Difference Between Class I, II and III –
Class II vs. Class III medical devices
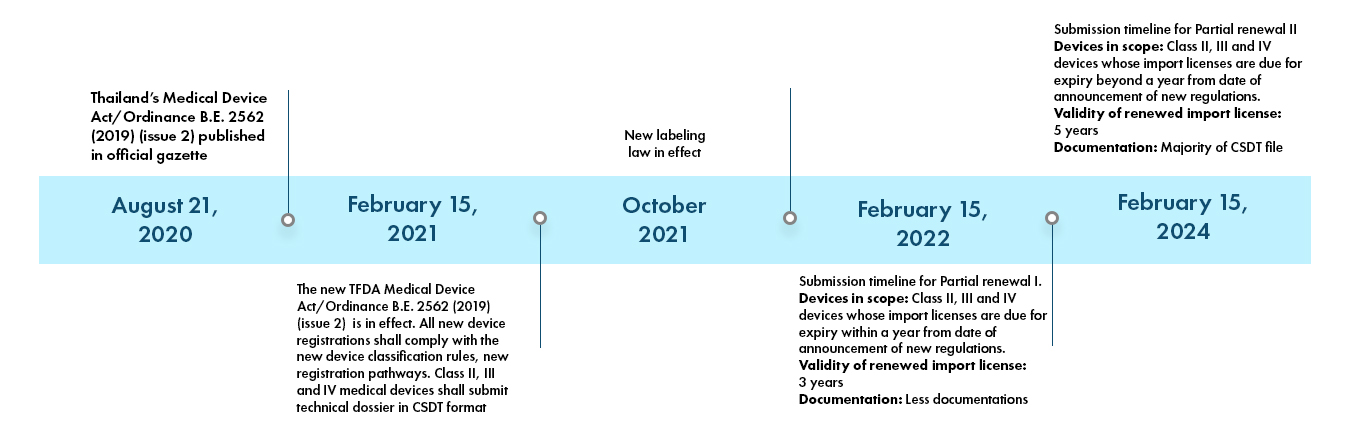
Thailand FDA Medical Device Registration, Thailand Local Authorized Representative
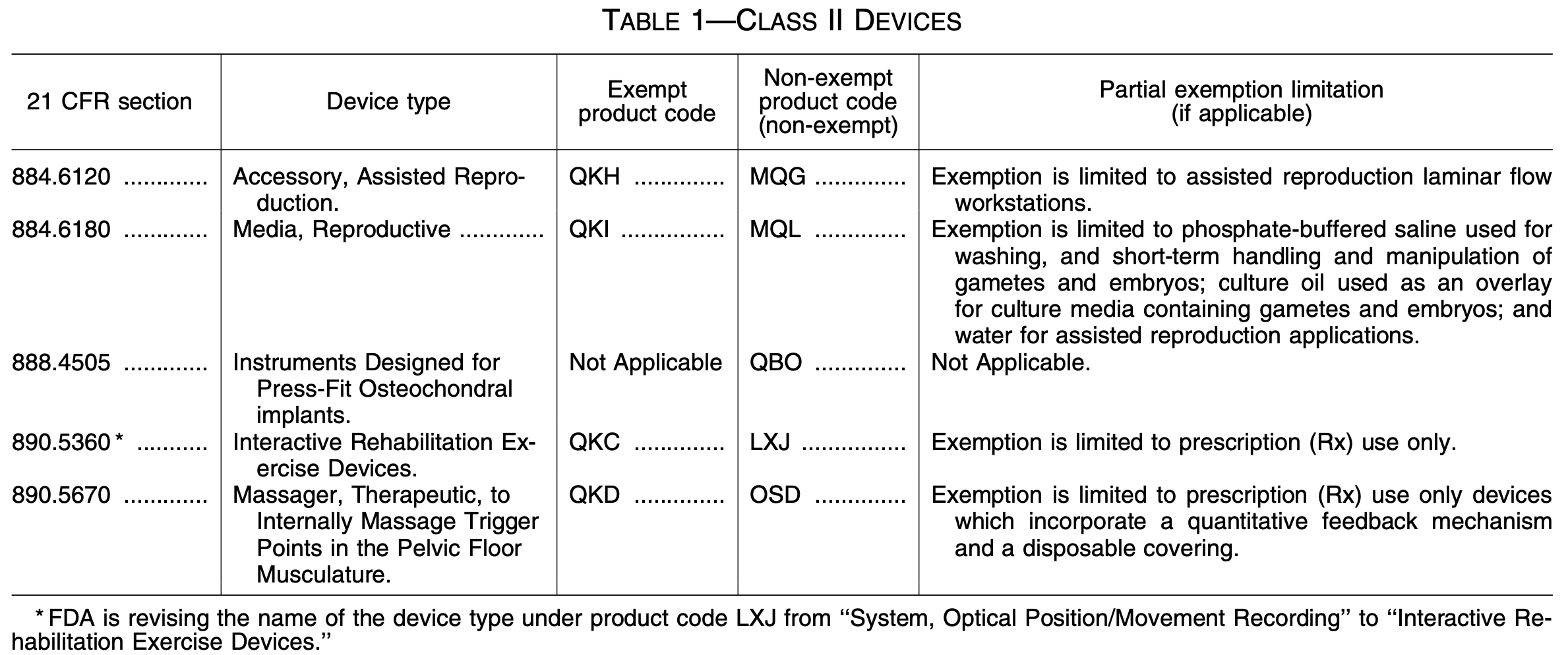
New 510(k) Exemptions for Class II Devices - SoftwareCPR
The 3 FDA medical device classes: differences and examples explained
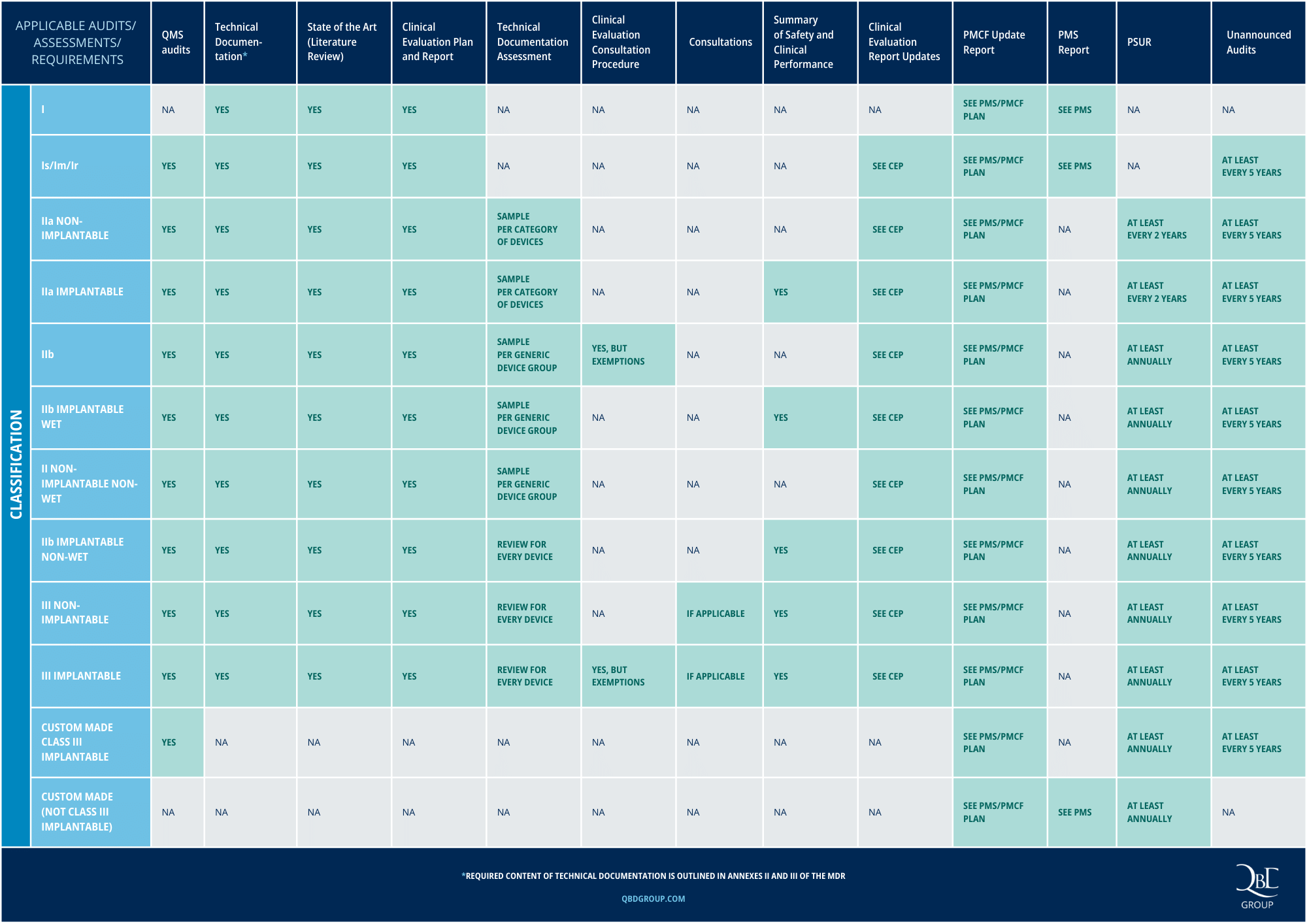
CE Approval for Medical Devices under MDR: key requirements per device class
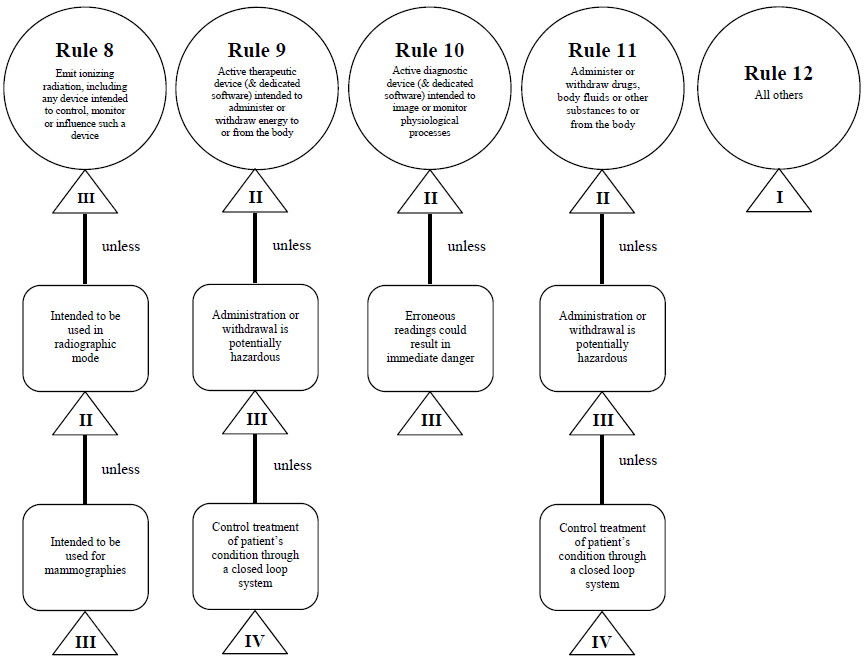
Guidance Document - Guidance on the Risk-based Classification System for Non-In Vitro Diagnostic Devices (non-IVDDs)

New world order 2013