
Compressibility factor, Z of a gas is given as Z= frac { pV }{ nRT
4.5 (384) In stock

4.5 (384) In stock
Click here:point_up_2:to get an answer to your question :writing_hand:compressibility factor z of a gas is given as z frac pv nrt
Click here👆to get an answer to your question ✍️ Compressibility factor- Z of a gas is given as Z- frac - pV - nRT - -i- What is the value of Z an ideal gas-ii- For real gas what will be the effect on value of Z above Boyle temperature

Physical Chemistry The Compression Factor (Z) [w/1 example]

Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2

The compressibility factor of a gas is defined as Z=PV/nRT. The compressibility factor of an ideal gas is:1-1zeroinfinite

Compressibility factor `Z=(PV)/(RT)`. Considering ideal gas, real gas, and gases at critical

Non-Ideal Gas Behavior Chemistry: Atoms First
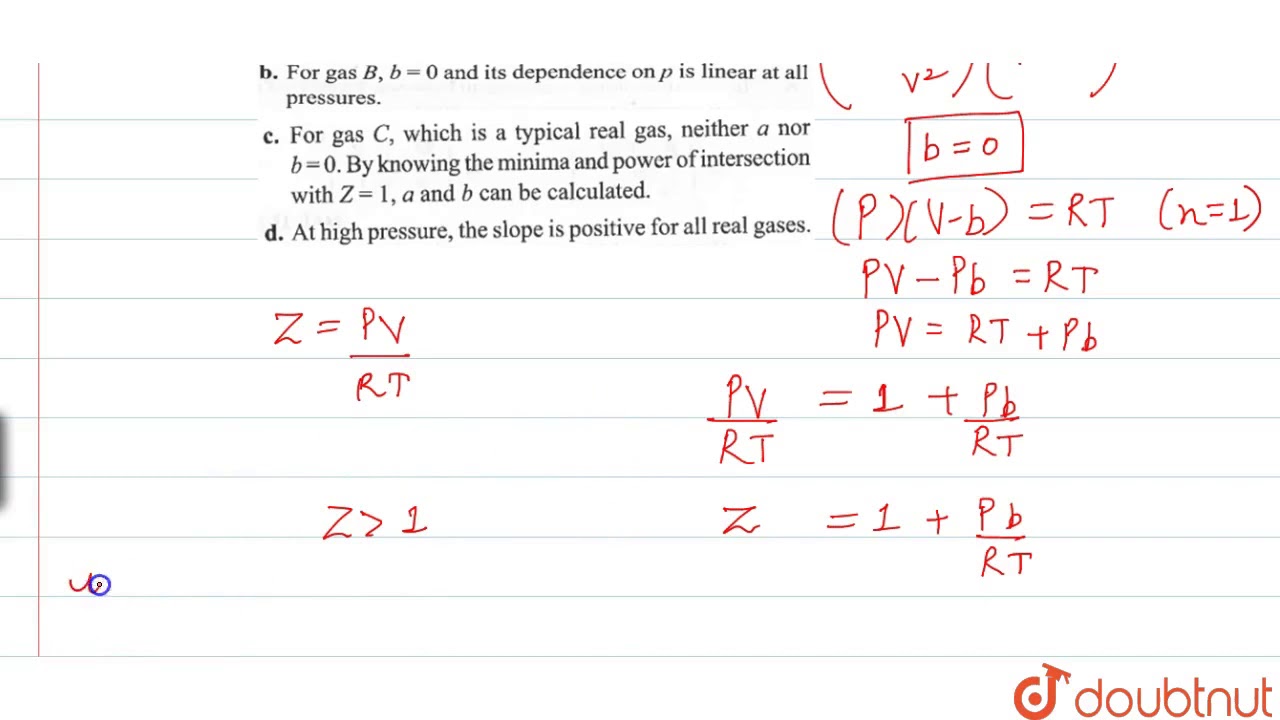
The given graph represents the variations of compressibility factor `Z=PV// nRT` vs `
What is the value of compressibility factor for a non-ideal gas? - Quora

1.7: Connecting the van der Waals and the viral equations: the Boyle temperature - Chemistry LibreTexts

1. The compressibility factor, z, is the ratio of

Compressibility factor of water vapor along its saturation curve. Error

COMPRESSIBILITY FACTOR