SOLVED: A 25 gram piece of hot metal at 97°C is plunged into a 35
5 (535) In stock
5 (535) In stock

Solved 31. A piece of metal weighing 418.6 grams was put
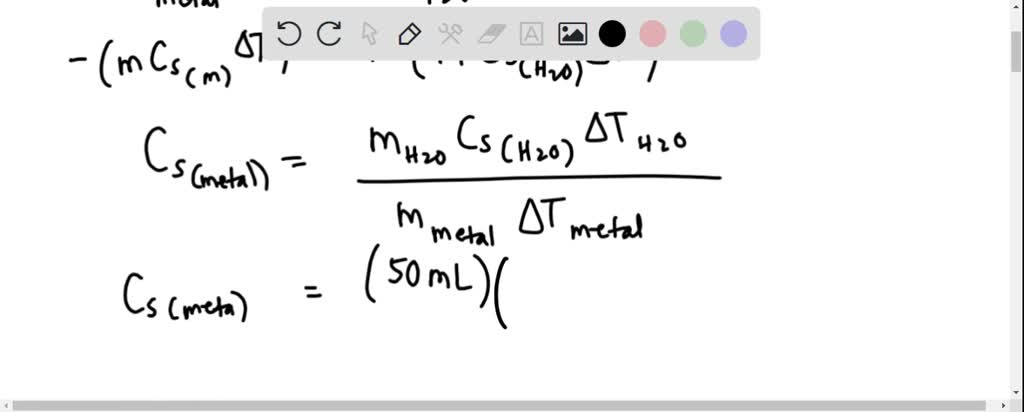
SOLVED: A sample of metal that has a mass of 12.48 g is heated to

Steel Times International November December 2022 by Quartz

Steel Times International November December 2022 by Quartz

SOLVED: Suppose a hot (89.5°C) piece of copper metal (c = 0.385 g K^-1) with a mass of 2.55 g is put into 50.0 g of water (c = 4.184 g K^-1).
The Gabe Collins Bookshelf: China Energy, Strategic Resources

PHYWE Solutions for Schools and Universities by PHYWE Systeme GmbH

Chapter 7 Thermochemistry - Pearson Canada
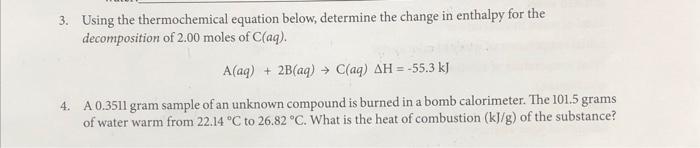
Solved Calculations Show the work for each step of the

AP Specific Heat (Final Temp. Metal Dropped into Water)

Electric Folding Mobility Tricycle – Liberty Trike

SOLVED: 4 250 sample of water with an initial temperature of 98.8
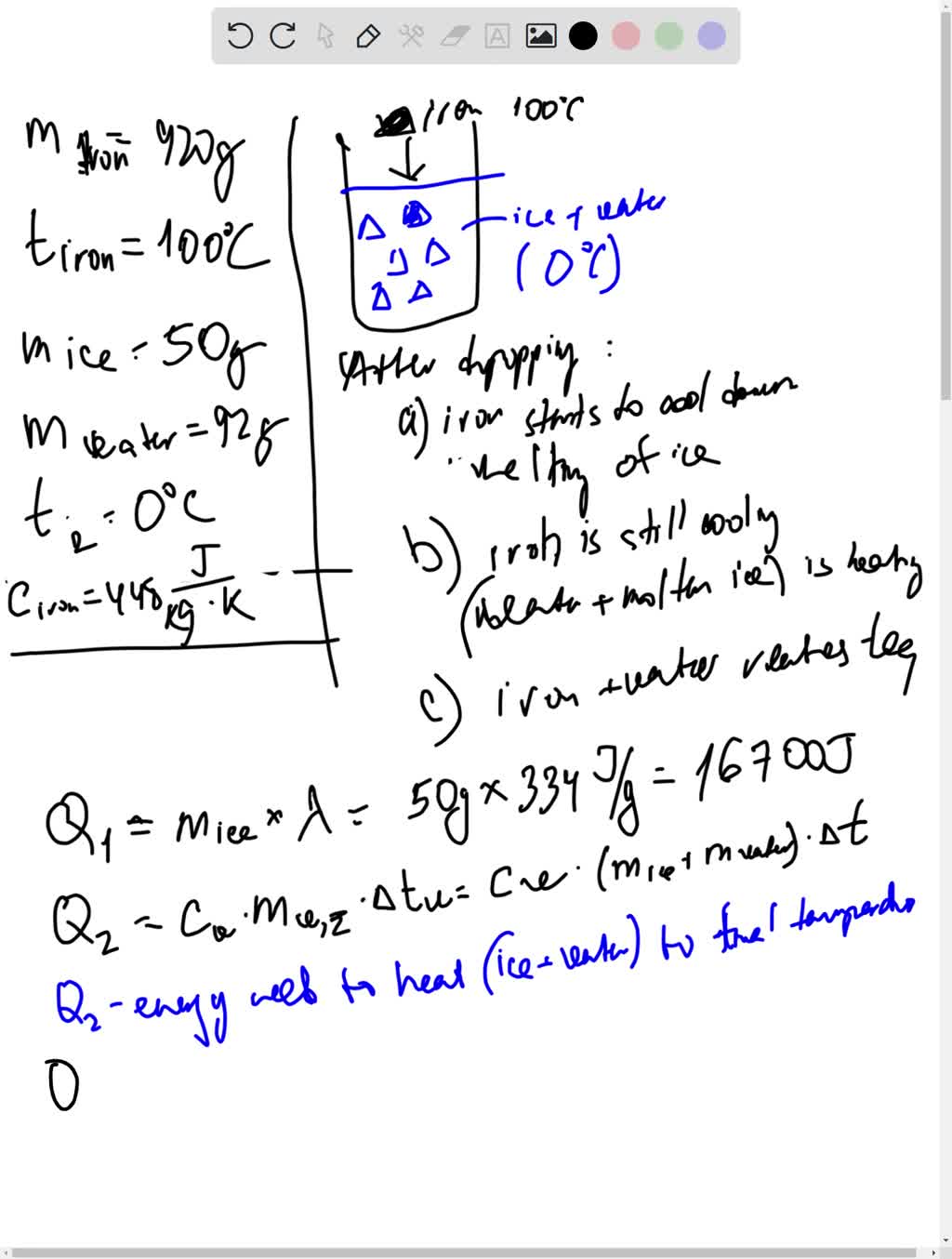
SOLVED: A 920g piece of iron at 100°C is dropped into a

5.22 A 70.0-g piece of metal at 80.0 °C is placed in 100 g of water at 22.0 °C contained in a

A 25.0 g sample of metal at 16.0 °C is warmed to 22.1 °C by 259 J