The entropy change for the conversion of 36 g water to vapour at
4.6 (752) In stock
4.6 (752) In stock
The entropy change for the conversion of 36 g water to vapour at its boiling point at 1 atm is (Enthalpy of vaporization for water is 40.63 kJ mol–1)
The entropy change for the conversion of 36 g water to vapour at its boiling point at 1 atm is -Enthalpy of vaporization for water is 40-63 kJ mol-1

66. The entropy change for the conversion of 36 g of water to vapour at 100°C (Normal boiling point) is

Latent heat of vaporization as a function of (a) salinity (at 20 °C and
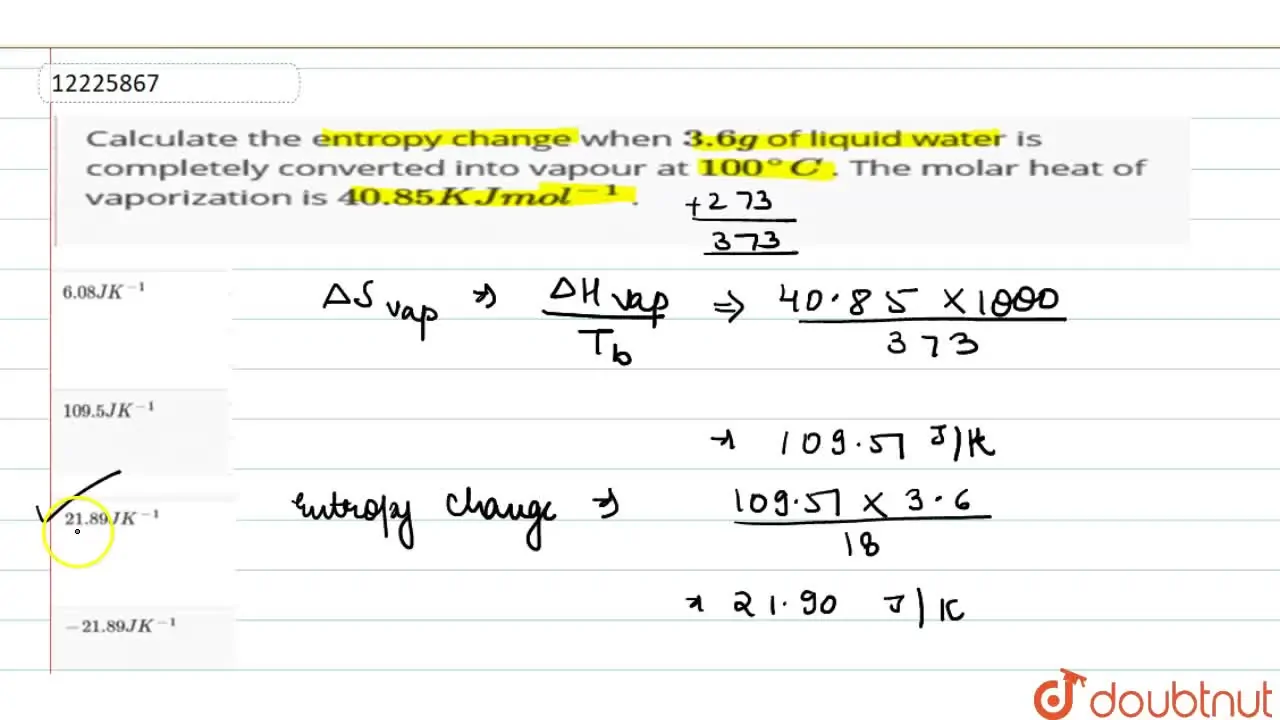
Calculate the entropy change when 3.6g of liquid water is completely c

The entropy change associated with the conversion of 1 kg of ice at 273 K to water vapours at 383 K

3) 6025 JAK (4) 602.5 JIK 87. Calculate the entropy change the conversion of 36 g water to vapour 373 K; AH, HO=40.63 k mor! (2) 202.07 JAK () 602 JK (4)

The entropy change involved in the conversion of 1 mole of liquid water at 373 K to vapour will be:Given: H vap =2.257 kJ / gA. 150 JK 1 mol 1B. 130.6

Energies, Free Full-Text

559) Calculate the entropy change when 3.6 g of liquid water is completely converted into vanours 373 K. The molar heat of vaporization of water is 40.85 kJ mol! b) 2.189 JK
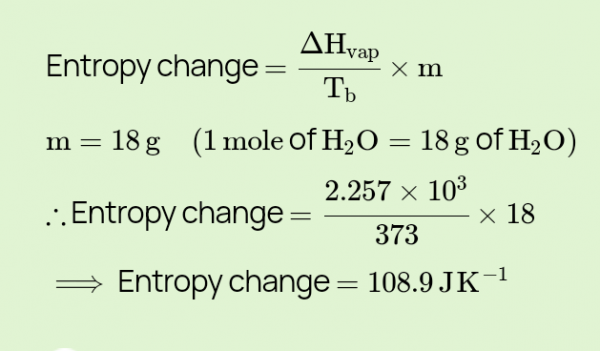
calculate the change in entropy for the conversion of one mole of liquid water to - Myschool

Isotonic separation enabled efficient low-grade heat conversion with thermal-responsive ionic liquids - ScienceDirect
Solubility - Wikipedia