
At a given temperature T gases Ne Ar Xe and Kr are found to deviate from ideal gas behavior (JEE MAINS 2019) - Doctor Logics Sunny Garg Chemistry
4.5 (301) In stock

4.5 (301) In stock
At a given temperature T, gases Ne, Ar, Xe and Kr are found to deviate from ideal gas behavior. Their equation of state is given as P=RTV−b at T. Here, b is the van der Waals constant. Which gas will exhibit steepest increase in the plot of Z (compression factor) vs P?

Problem 4: The ideal gas law describes the

At a given temperature T, gases Ne, Ar, Xe and Kr are found to deviate from ideal gas behaviour.
At a given temperature T, gases Ne, Ar, Xe and Kr are found to deviate from ideal gas behaviour. - Sarthaks eConnect

GK Theory + GK Quiz & Business Quiz + Business Compendium 1 - 202 Pages

At a given temperature T, gases Ne, Ar, Xe and Kr are found to deviate from ideal gas behaviour. Their equation of state is given as p = dfrac {RT}{V - b}
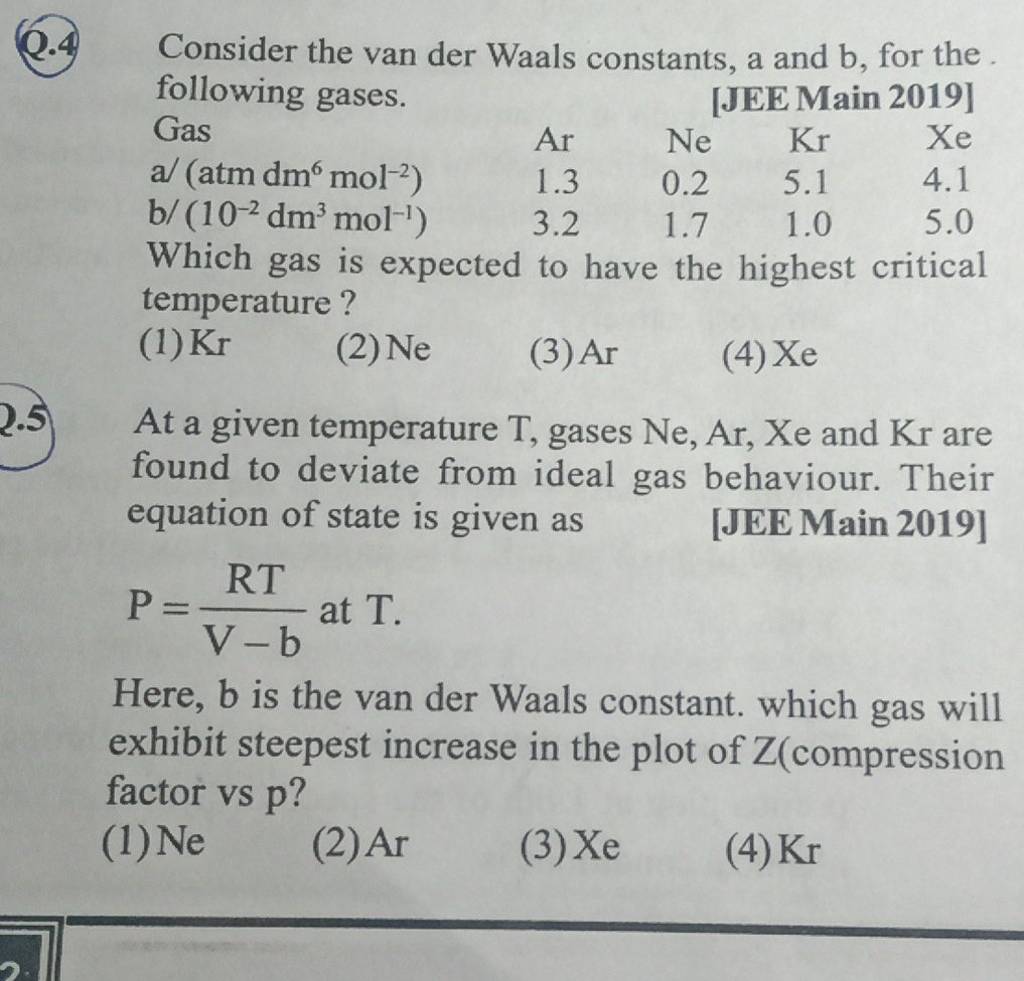
At a given temperature T, gases Ne,Ar,Xe and Kr are found to deviate from..

At a given temperature T, gases Ne, Ar, Xe and Kr are found to deviate

Recent Trends in Mechanical Engineering: Select Proceedings of PRIME 2021 9811977089, 9789811977084

Competition Science Vision - February 2008, PDF

At a given temperature T gases Ne Ar Xe and Kr are found to deviate from ideal gas behavior (JEE MAINS 2019) - Doctor Logics Sunny Garg Chemistry