
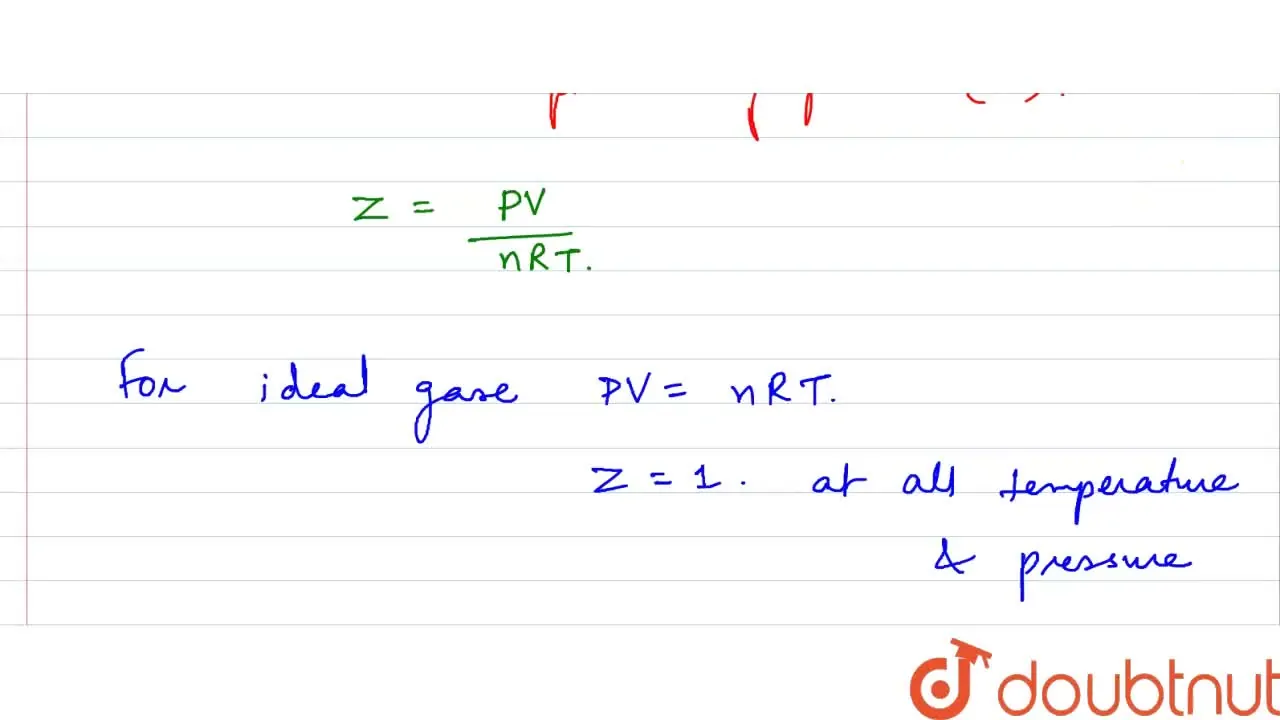
The compressibility factor Z for an ideal gas will be
4.8 (563) In stock

4.8 (563) In stock
The compressibility factor Z for an ideal gas will be
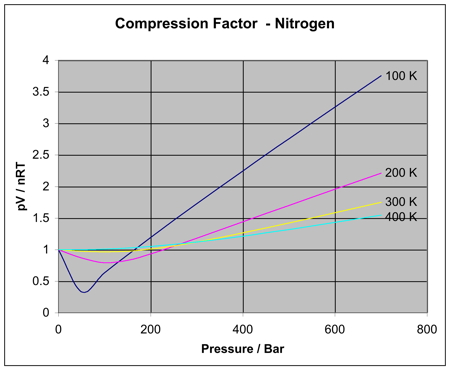
Non-ideal behavior of gases (article)

Marathi] The compressibility factor of a gas is defined as z = PV //R
Gujrati] Explain compressibility factor (Z).

Compressibility factor (gases) - Citizendium

Real Gases vs Ideal Gases & the Compressibility Factor

Which of the following statements is/are correct? (a) all real gases are less compressible

The compressibility factor of a gas is defined as z = PV //RT . The co
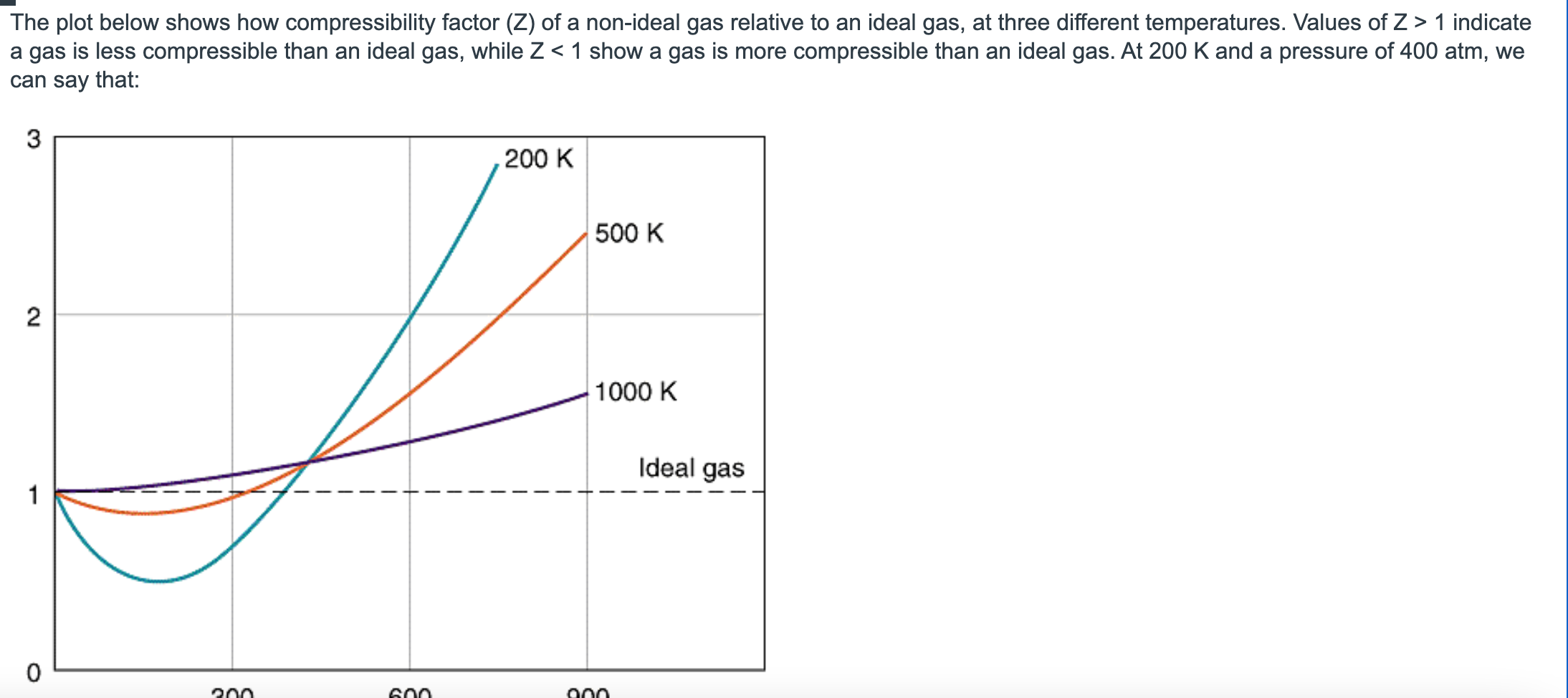
Solved The plot below shows how compressibility factor (Z)
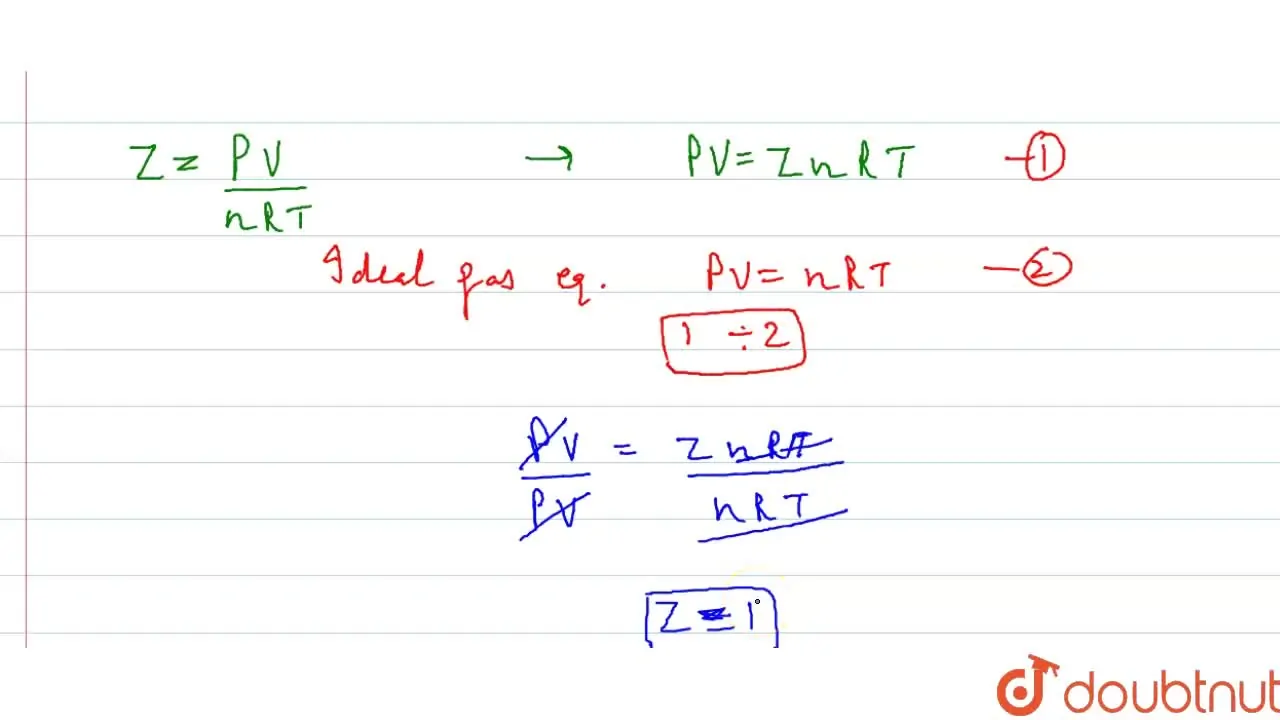
Punjabi] (True/False) The compressibility factor (z) for ideal gases

Compressibility factor (z): real gases deviate from ideal behav-Turito

Compressibility factor, Z of a gas is given as Z=(pV)/(nRT) (i) What
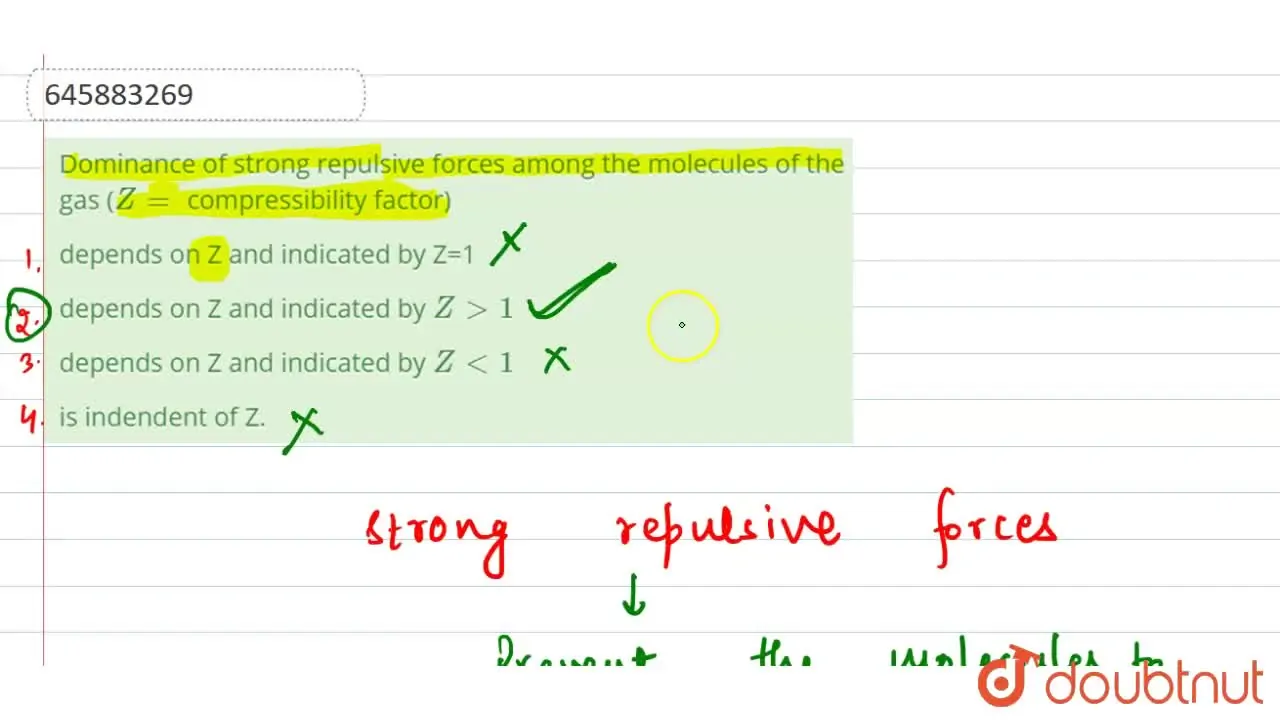
Dominance of strong repulsive forces among the molecules of the gas

Z gt 1 and repulsive forces are dominant.
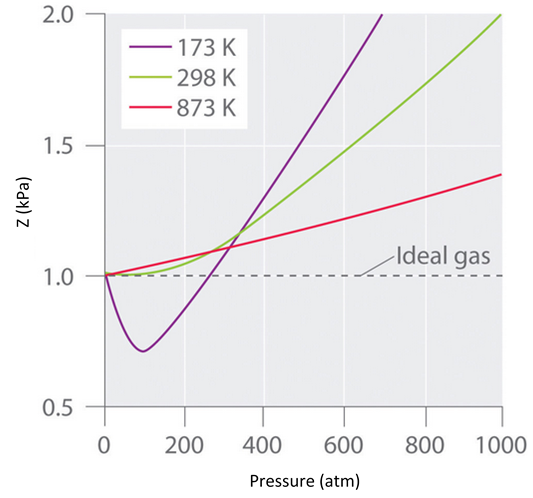
4.2: Real Gases (Deviations From Ideal Behavior) - Chemistry LibreTexts