
At 300 K, 36 g of glucose present per litre in its solution has an osm
4.8 (107) In stock

4.8 (107) In stock
pi=CRT" (C = molar concentration)" (pi(1))/(pi(2))=(C(1))/(C(2))," "(4.98)/(1.52)=(36//180)/(C(2))" or "C(2)=(36)/(180)xx(1.52)/(4.98)="0.061 M"

Lecture Notes: Chapter 1-Science and Measurements

PHRM2022 Dosage Form Design A2, PHRM2022 - Dosage Form Design A2 - UQ

Find the molaity of water. Given: rho =1000kg//m^(3) [Report your

General Tests, Processes and Apparatus

ANSWERED] elevation constant 18 The molar mass of a non volatile - Kunduz
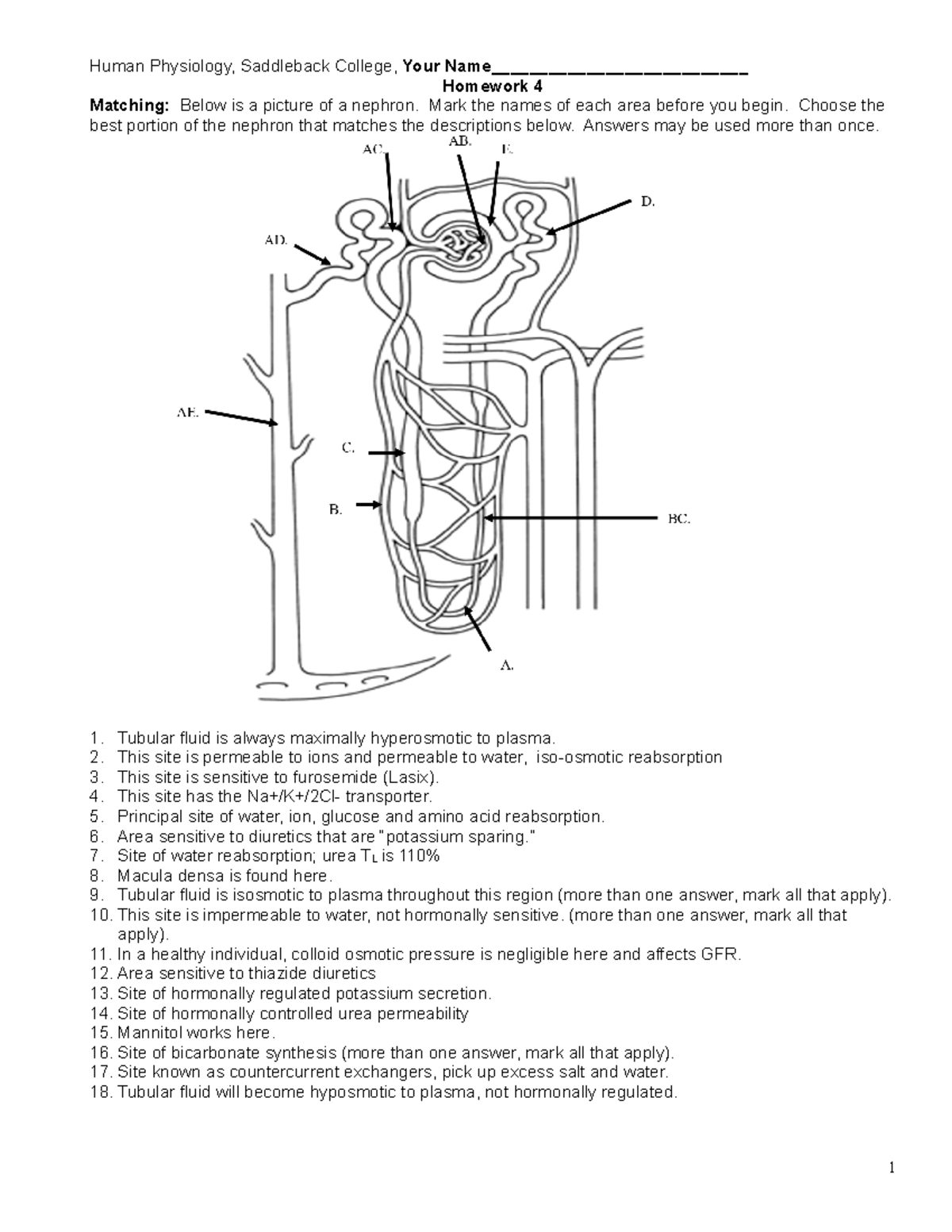
Lecture Unit 4 - HW Packet 4 - Human Physiology, Saddleback College, Your - Studocu

30 g of glucose present per litre has an osmotic pressure of 4.91 atm 303 K. If the osmotic pressure of the same solution is 1.5 atm the same tempera- ture, what

Lab exam 1.pdf - VTPP 423 PHYSIOLOGICAL MEASUREMENTS Date 1/28/21 GOHIL AISHWA Name Lab Station 9A Kendall Amir Lab Partners

29394

If the elevation in boiling point of a solution of 10 g of solute (mol