At Critical Temperature,pressure and volume . The compressibility
4.6 (107) In stock
4.6 (107) In stock

Math Physics Chemistry Questions Discussion Lists - Dated: 2020-12-02

If excluded volume is taken zero, compressibility factor Z is

States of Matter, PDF, Gases

States of Matter, PDF, Gases
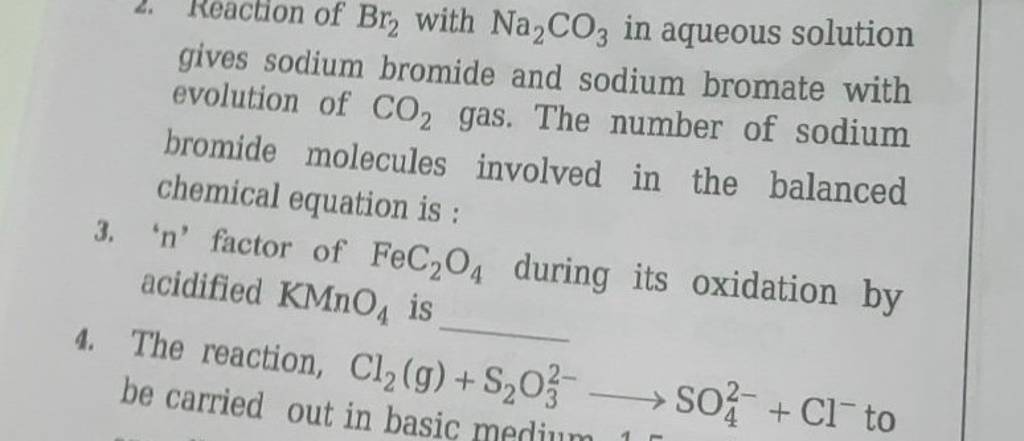
Filo Student Questions For CBSE , Class 11 , Chemistry , Gase

the compressibility factor for o2 is 0.308 and its critical pressure and critical volume are 50.1atm and

SOLVED: at critical temperature, pressure and volume the compressibility factor z is?
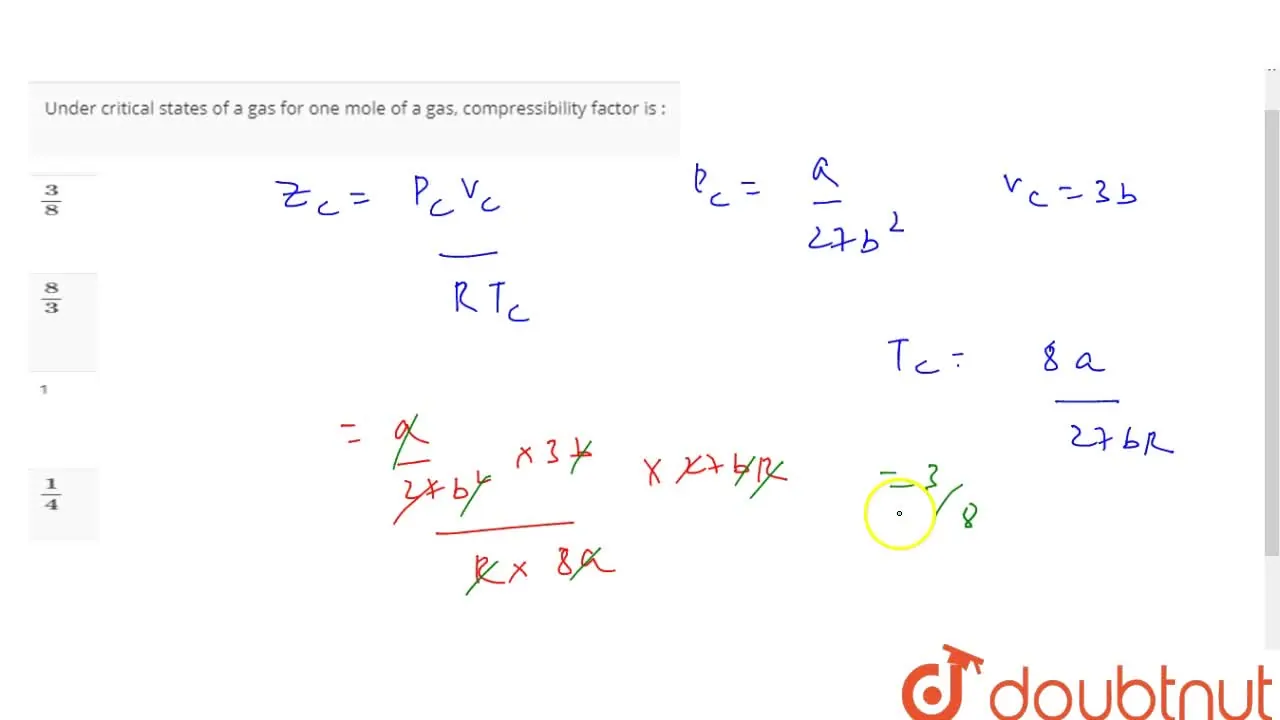
Under critical states of a gas for one mole of a gas, compressibility

Telugu] The compressibility factor (Z) of a gas at critical state is

Odia] Define critical temperature, critical pressure and critical vol

States of Matter study material with practice question 2023 - Chapter Contents Intermolecular - Studocu