
At high pressure, the compressibility factor 'Z' is equal toa
4.5 (496) In stock

4.5 (496) In stock

NEET Chemistry Chapter Wise Mock Test - Mock Test 2 - CBSE Tuts

a) Change in Z-factor of CO2 versus pressure and at constant

REAL GASES, DEVIATION FROM IDEAL GAS BEHAVIOUR

If a gas gets half compressed, compared to an ideal gas, the compressi

Variation OF compressibility factor with pressure

physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange
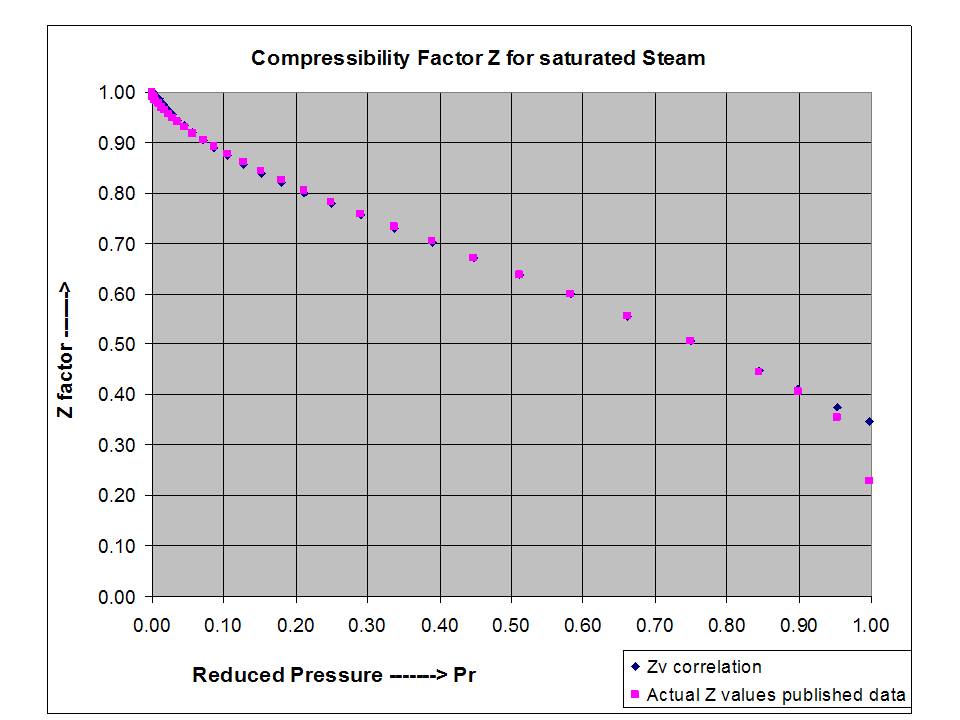
Compressibility factor Z

Gas Compressibility - an overview

At high pressure, the compressibility factor 'Z' is equal toa
At low pressure, the van der waal's equation is written as (P+ a/V^2)V=RT . Then compressibility factor is then equal to

2.8 – Real/Non-Ideal Gas Behaviours – General Chemistry for Gee-Gees

Gas Compressibility - an overview

NEET Chemistry Chapter Wise Mock Test - Mock Test 2 - CBSE Tuts